Pharmaceutical Industry
Digitalization for your auditability and traceability needs
Control your data flows and guarantee their integrity, facilitate the auditability of your Pharmaceutical Quality System, support your external growth policy, and develop information continuity throughout the value chain with your partners, thanks to a unique, scalable data platform: Phoenix.
Demanding study processes, auditability, marketing authorization (MA)…: drug regulation is constantly increasing. Any failure to comply with Good Manufacturing Practices during an inspection by an authority has an immediate impact on your sales and profitability. But you still need to innovate and react!
As a pharmaceutical laboratory or manufacturer, you operate within an extremely strict framework. Your profitability and competitiveness imperatives are driving you to re-evaluate your strategy, particularly in terms of digitalization and control of your information flows.
This is a major challenge, as your processes are increasingly cross-functional: research & development, quality, regulatory, purchasing, production, logistics, human resources… but also open to external partners. What’s more, if you have a large number of applications, the cornerstone of your Information System lies in the links and exchanges between all these tools, to serve your business processes.
The challenges of evolution and digitalization
in the pharmaceutical industry
XEVMPD (Extended EudraVigilance medicinal product dictionary), UDI unique codification, ISO/CEI 17025, CFR, Data Integrity… to meet regulatory and GMP/GxP requirements, efficient and verified management of data and information flows is key!
How do you offer exhaustive traceability?
Nomenclature, naming, version… You need to be able to reconstitute the product repository at any date and trace all changes.
Control data throughout the product lifecycle
Controlling data throughout the product lifecycle means having an MDM solution connected to all your IS applications.How can we improve interoperability with logistics partners?
Whether you’re a distributor or a carrier, your information systems need to be bidirectionally connected. You need an immediate view of delivery status at all times, so you can anticipate and publish information to end customers…Structuring communications with external information systems
The complementary nature of IS urbanization and the exposure of services and data through API Management means you can share information with your partners’ systems seamlessly and securely.
How can I promote my supply chain expertise to my partners and customers?
Your mastery of the supply chain and the wealth of information you gather constitute a competitive advantage that deserves to be fully exploited.Offering complementary services
By integrating the IoT and exposing selected facets of your information system, you can offer complementary services, transparency and visibility, all of which are assets in the eyes of your customers! This is the API Management dimension.How do you manage external exchanges?
You increasingly need to open up your Information System to your partners and subcontractors (subsidiaries, laboratories, startups…), while controlling every exchange and every piece of data in transit.Computerize exchanges with the ecosystem
The combination of an application bus within your IS and API Management to expose data secures information sharing and supports the implementation of cross-functional flows between your IS and those of your partners.







What are the methods and solutions for meeting the challenge of digitizing the pharmaceutical industries?
Pharmaceutical companies generally have a large number of high-level application bricks in their Information Systems. However, while this best-of-breed approach to each type of software (WMS, LIMS, MES, CRM, ERP…) is a first response to your challenges, it’s not enough!
Auditability of your Information System, total traceability of flows and compliance with standards require you to urbanize your entire software estate and implement cross-application processes, both internally and externally.
While IS urbanization is primarily justified by quality and standards imperatives, it’s also an opportunity to take your digital transformation to the next level, and improve your productivity and agility!
Phoenix data platform to support the digitization of the pharmaceutical industry
We’ve been supporting the pharmaceutical industry in its digital transformation for many years. We are fully aware that IT tools are only one way of meeting your challenges:
A data exchange environment
which is standardized and controlled
Eliminate the risk of errors
(re-entries, validation, process steps, real-time updates...)
Supervise all exchanged flows
internally and externally
Meeting auditability requirements
and compliance with standards and procedures
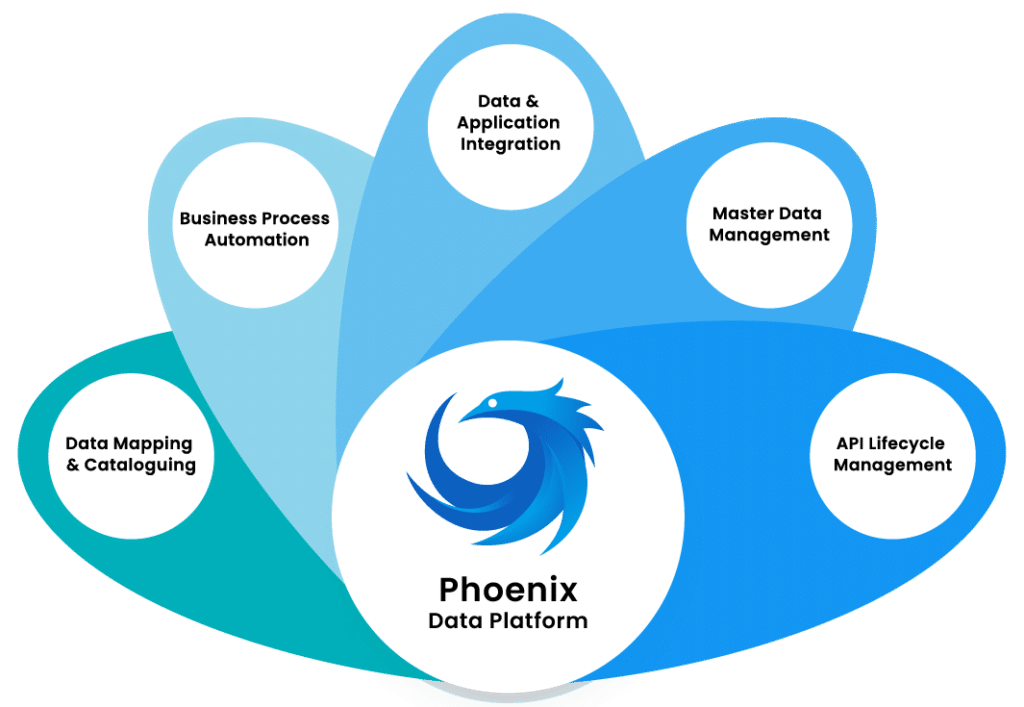
The Phoenix platform: a 360° response to the data challenges faced by manufacturers and pharmaceutical companies
Blueway’s software platform offers you a complete solution, calibrated for the pharmaceutical industry, to meet your critical flow and process control challenges. It integrates all dimensions of data exchange (BPM, MDM, ESB and API Management). Processes are documented, and tests are reproducible in the event of a problem.
Full-web, available in PaaS and On-Premise, our integrated Phoenix platform connects natively with the main software packages used in the pharmaceutical industry (WMS, LIMS, ERP, MES…). You benefit from a 360°, real-time view of your processes, as well as supervision tools to alert you as soon as an incident occurs, and to monitor your performance indicators.

Use cases
“With Phoenix, I strengthened the scalability of my Information System to support my external growth policy and the integration of new systems.”
“My Product MDM is now a central element to help me comply with Good Manufacturing Practices.”
“Blueway’s BPM approach connected to my business applications reinforces the agility of my pharmaceutical processes while facilitating their auditability.”
“We control exchanges with our ecosystem of start-ups and laboratories and capitalize on shared data to control end-to-end flows.”
about our customers' projects in pharmaceutical industry?
Frequently asked questions about digitalization in the pharmaceutical sector
To facilitate the integration of new entities, it is crucial to adopt a strategy of information system urbanization and harmonization of processes and data governance. In terms of tools, this involves implementing an Enterprise Service Bus (ESB) to drive inter-application communication, a Master Data Management (MDM) approach for unified data governance and management, and Business Process Management (BPM) to align and optimize operational processes across the group.